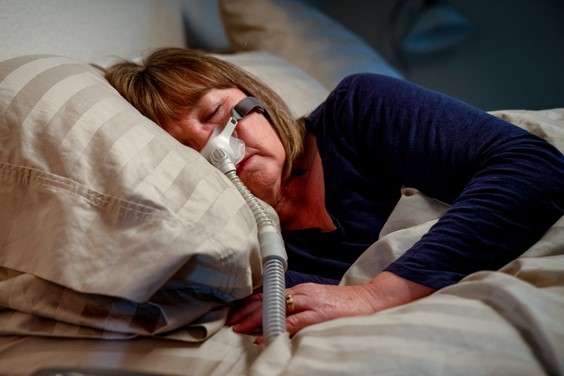
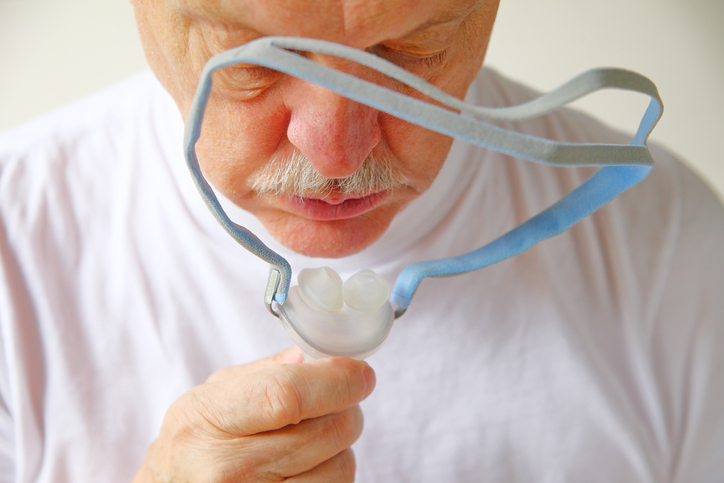
On October 8, 2021, the Judicial Panel on Multidistrict Litigation (“JPML”) created a new MDL (“multidistrict litigation”) for federal lawsuits for individuals who have suffered due to Philips’ recalled Continuous Positive Airway Pressure (CPAP), Bi-Level Positive Airway Pressure (Bi-Level PAP), and mechanical ventilator devices, which contain polyester-based polyurethane (PE-PUR) sound abatement foam that may degrade...
Philips Recalled CPAP/Bi-Level PAP Litigation Update: Federal Cases Centralized in MDL in Western District of Pennsylvania
Categories: Philips CPAP and BiPAP Recall