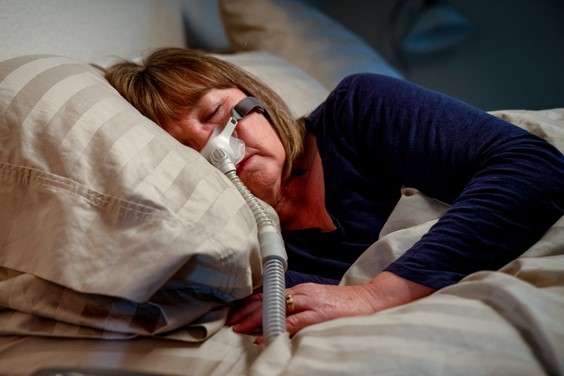
On October 8, 2021, the Judicial Panel on Multidistrict Litigation (“JPML”) created a new MDL (“multidistrict litigation”) for federal lawsuits for individuals who have suffered due to Philips’ recalled Continuous Positive Airway Pressure (CPAP), Bi-Level Positive Airway Pressure (Bi-Level PAP), and mechanical ventilator devices, which contain polyester-based polyurethane (PE-PUR) sound abatement foam that may degrade into particles or off-gas volatile organic compounds that may then be ingested or inhaled by the user, causing injury. Certain devices were recalled in June, 2021. The Plaintiffs/Claimants allege that Defendants Philips North America LLC and Philips RS North America LLC (“Philips”) concealed the problems with the PEPUR foam before the recall was announced and made misrepresentations regarding the recalled devices in connection with their marketing and sales. At the time of the JPML’s 10/8/21 order, it reported 104 cases filed in 31 districts.
An MDL is a type of legal proceeding that helps federal courts efficiently manage many similar cases filed in many different federal courts across the U.S. by allowing the temporary transfer of all the federal civil lawsuits to one or more district courts for pretrial consolidation or coordination. The JPML is a group of federal judges designated by the Chief Justice of the United States, which has the responsibility for determining which cases qualify for MDL treatment as well as which district court to transfer and consolidate these cases.
In its October 8, 2021 order, the JPML determined that the Philips CPAP cases involve common questions of fact, and that centralization will serve the convenience of the parties and witnesses and promote the just and efficient conduct of this litigation. Specifically, it noted that the Philips actions raise similar factual questions regarding the recalled devices and the conduct of the recall and will require common discovery regarding the development and safety of the recalled devices and the potential harm that can be caused by the alleged defect. The JPML determined that centralization will eliminate duplicative discovery; prevent inconsistent pretrial rulings; and conserve the resources of the parties, their counsel, and the judiciary. The JPML also noted that the recalled products were primarily manufactured by Philips RS North America LLC (formerly Philips Respironics) in Murrysville, Pennsylvania. Thus, many of witnesses and much of the documentary evidence relevant to this litigation likely will be located within the Western District of Pennsylvania.
The JPML assigned the docket to the Honorable Joy Flowers Conti, Senior District Judge in the Western District of Pennsylvania, an experienced transferee judge, noting that it was confident Judge Flowers Conti would steer this litigation on a prudent and expeditious course.
Borgess Law, LLC will continue to keep you updated on this litigation.
How We Can Help
If you or someone you love suffered injury due to a recalled Philips BiPAP/CPAP device you should be aware of the ongoing litigation.
Attorney Pamela A. Borgess, the founder of Borgess Law, LLC, has extensive experience with numerous national high-profile defective medical devices cases and can discuss your legal options. For more information about the Philips CPAP litigation or to discuss a potential claim, contact Borgess Law at (567) 455-5955. You can also contact Borgess Law by submitting an online inquiry. Borgess Law never charges for initial consultations. We welcome any questions you may have.