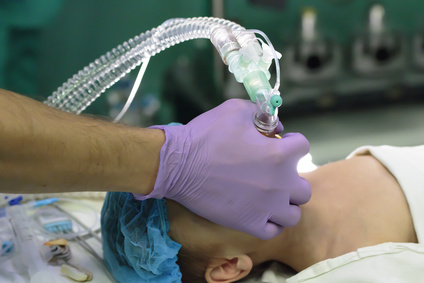
Today, the FDA issued a drug safety communication that it has approved previously announced label changes regarding the use of general anesthetic and sedation medicines in children younger than 3 years. These changes include: A new Warning stating that exposure to these medicines for lengthy periods of time or over multiple surgeries or procedures may...
FDA Issues Drug Safety Communication Regarding Use of General Anesthetic and Sedation Drugs in Young Children