On March 31, 2017, Mylan N.V. announced that Meridian Medical Technologies, a Pfizer company and Mylan’s manufacturing partner for EpiPen® Auto-Injector, expanded its recall of select lots of EpiPen (epinephrine injection, USP) and EpiPen Jr® (epinephrine injection, USP) Auto-Injectors to now include additional lots distributed in the U.S. and other markets.
As reported in the announcement posted by the FDA, the potential defect could make the device difficult to activate in an emergency (failure to activate or increased force needed to activate) and have significant health consequences for a patient experiencing a life-threatening allergic reaction (anaphylaxis).
The recalled product was manufactured by Meridian Medical Technologies, a Pfizer company, and distributed by Mylan Specialty between December 2015 and July 2016. The expanded voluntary recall is being initiated in the U.S. and also will extend to additional markets in Europe, Asia, North and South America.
The recall impacts the 0.3 mg and 0.15 mg strengths of EpiPen Auto-Injector. None of the recalled lots reportedly include the authorized generic for EpiPen Auto-Injector, which is also manufactured by Meridian Medical Technologies.
U.S. Impacted Lots:
| Product/Dosage | NDC Number | Lot Number | Expiration Date |
| EpiPen Jr 2-Pak® Auto-Injectors, 0.15 mg | 49502-501-02 | 5GN767 | April 2017 |
| EpiPen Jr 2-Pak® Auto-Injectors, 0.15 mg | 49502-501-02 | 5GN773 | April 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 5GM631 | April 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 5GM640 | May 2017 |
| EpiPen Jr 2-Pak® Auto-Injectors, 0.15 mg | 49502-501-02 | 6GN215 | September 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 6GM082 | September 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 6GM072 | September 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 6GM081 | September 2017 |
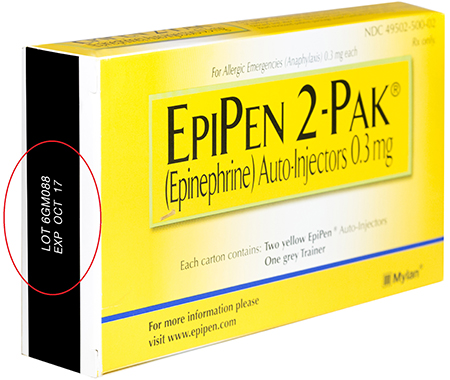
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 6GM088 | October 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 6GM199 | October 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 6GM091 | October 2017 |
| EpiPen 2-Pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 6GM198 | October 2017 |
| EpiPen 2-pak® Auto-Injectors, 0.3 mg | 49502-500-02 | 6GM087 | October 2017 |
Attorney Pamela A. Borgess, the founder of Borgess Law, LLC, has successfully represented multiple clients who have sustained serious injury or whose loved ones have died as a result of defective or recalled products, including recalled medical devices and drugs. Attorney Borgess will happy to discuss your legal options. For more information about the recall, or to discuss a potential claim, contact Borgess Law at (567) 455-5955 or toll-free at (844) LAW-9144. You can also contact Borgess Law by submitting an online inquiry. Borgess Law never charges for initial consultations. We welcome any questions you may have.